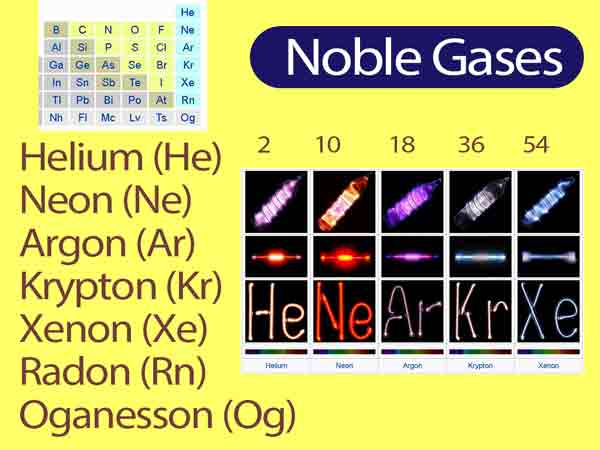
Describe the Properties of Noble Gases
They find uses as inert atmospheres neon signs and as coolants. The noble gases are a group of non-metallic chemical elements that under normal conditions of pressure and temperature are monoatomic odorless colorless gases.

What Are Noble Gases Properties And Characteristics Of Noble Gases Video Lesson Transcript Study Com
The members of this group are helium neon argon krypton xenon and radon.
. Properties of Helium He Helium is a chemical element which is denoted by the. Physical Properties of Noble Gasses At room temperature and pressure all the elements of group 18 exist in a gaseous state. Do not include the phase designation in your response.
In the carbon dioxide cycle carbon dioxide is taken in by plants and used in photosynthesis. It is also used in the production of titanium. Based on their electronic arrangement explain whether they can exist alone in nature.
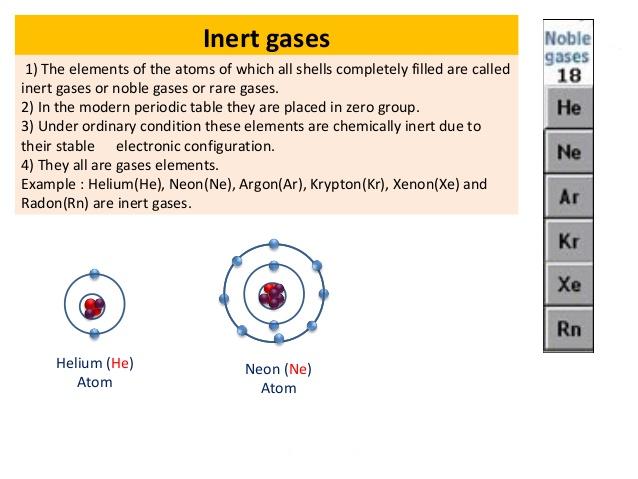
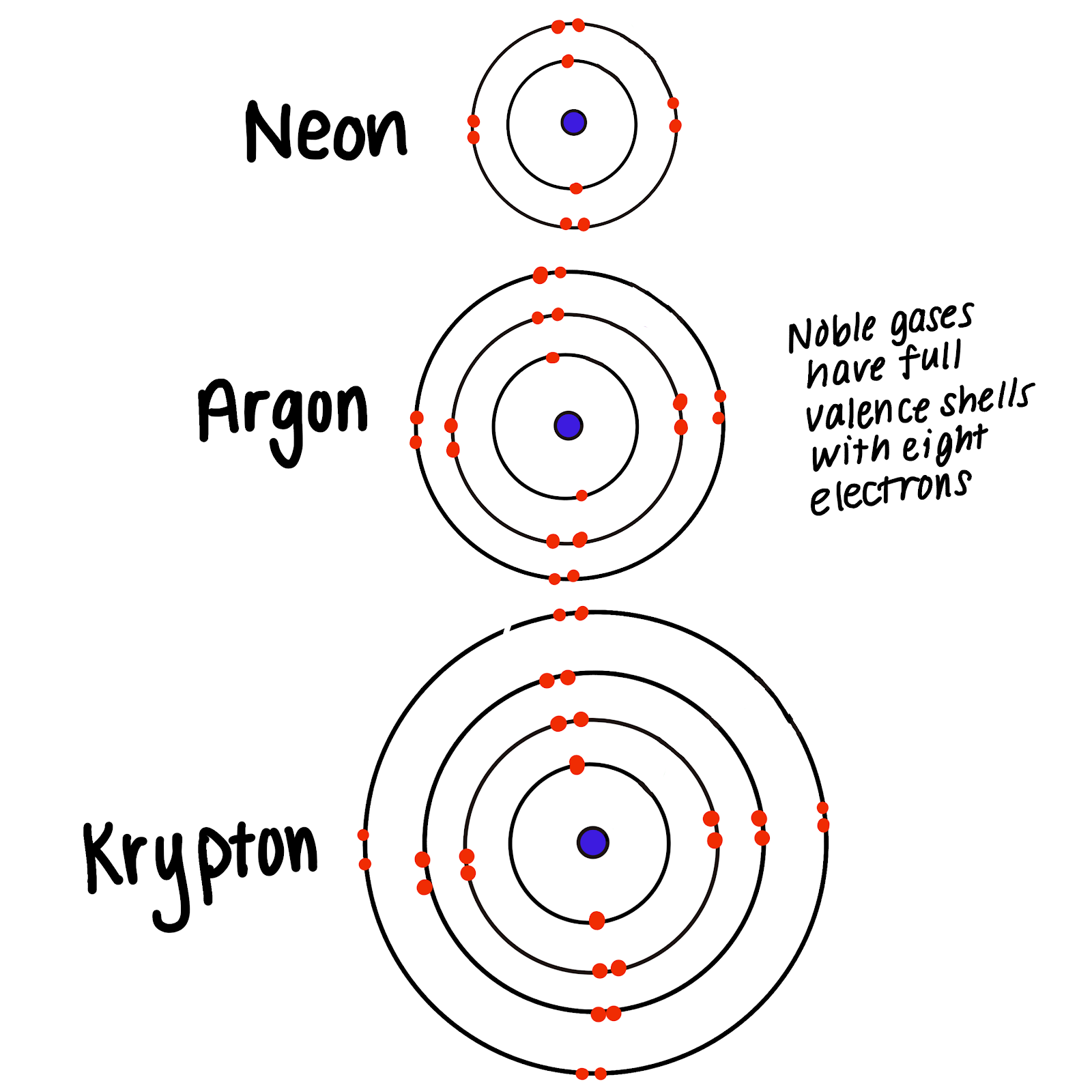
This is the reason behind the chemically inert nature of the group 18 elements. They are placed in group 18 of the periodic table. Their electronic configuration reveals that atoms of these elements have completely filled orbitals.
Properties of noble gases. Describe the properties of noble gases. Properties of Noble Gases Noble gases are relatively non-reactive in nature a property which can be attributed to their full or complete outer shell of valence electrons which gives them very little or absolutely no ability to indulge in chemical reactions.
The electronic configuration of noble gases is given as. Atoms of group 1 and 7 elements have incomplete outer shells so they are reactive atoms of group 0 elements have complete outer shells so they are unreactive. Air contains nitrogen 78 oxygen 21 carbon dioxide 004 and rare gases less than 1.
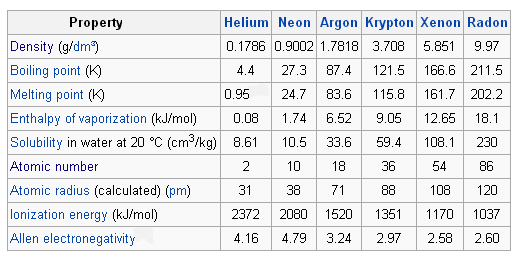
A limited amount of argon is used in germanium and silicon crystals. Noble gas any of the seven chemical elements that make up Group 18 VIIIa of the periodic table. I All the nobie gases are colorless and odorless I Practically insoluhie in water I Low boiling and melting points as they exist as monoatomic entities and very weak intermolecular interactions are present within the gas particles I They follow the normal group trends in the periodic table ie.
They have a full outer shell and cannot live alone In nature as they cannot bind with other gases. Uses of Noble Gases. The noble gases have very weak intermolecular forces and therefore have very low melting and boiling points.
They traditionally have been labeled Group 0 in the periodic table because for decades after their. Based on their electronic arrangement explain whether they can exist alone in nature. Increase in the atomic radius along the group as we go down.
The correct answer was given. The three heaviest noble gases react with fluorine to form fluorides. They occur in low concentrations in the atmosphere.
They have very low melting and boiling points due to low. All the noble gases are monoatomic. Therefore the outermost valence shells of the noble gases can be considered as full.
The general electronic configuration of the noble gases can be written as ns 2 np 6. It also contains water dust bacteria and other materials. This inert atmosphere plays an important role in welding titanium aluminium stainless steel and magnesium.
All consist of monatomic. The elements are helium He neon Ne argon Ar krypton Kr xenon Xe radon Rn and oganesson Og. They are all colorlessodorless gases.
2 question Describe the properties of noble gases. The atmosphere supports life via two cycles. The noble gases are colourless odourless tasteless nonflammable gases.
Noble gases or otherwise called inert gases are stable and non reactive or more correctly they are less reactive. In metallurgical processes argon is widely used in order to provide the necessary inert atmosphere. The most significant property of the noble gases group 18 is their inactivity.
The melting and boiling point of all the Noble Gasses is very low due to the following reasons. Describe the properties preparation and uses of the noble gases Question Write the chemical formula of the noble gas that has two valence electrons. The properties of noble gases are.

Noble Gases Trends And Patterns Scienceaid

Group 0 18 Noble Gases Physical Properties Uses Helium Neon Argon Krpton Xenon Radon Melting Points Boiling Points Atomic Radii Density Inertness Explained Gcse Chemistry Ks4 Science Igcse O Level Revision Notes

Group 18 Elements Noble Gases Ppt Video Online Download

Chemistry Of Noble Gases Ppt Download

Noble Gases The Gases In Group 18 Properties Of Matter Chemistry Fuseschool Youtube

What Are Noble Gases Properties And Characteristics Of Noble Gases Video Lesson Transcript Study Com

Noble Gases Trends And Patterns Scienceaid
What Do All Of The Noble Gases Have Socratic
The Properties Of The Noble Inert Gases Science Online

Noble Gases Video Jacksonville High

The Noble Gases Group 18 Introduction To Chemistry

Noble Gases Trends And Patterns Scienceaid

Why Are Called Noble Gases What Are The Properties Of Noble Gases
The Noble Gases Group 18 Introduction To Chemistry
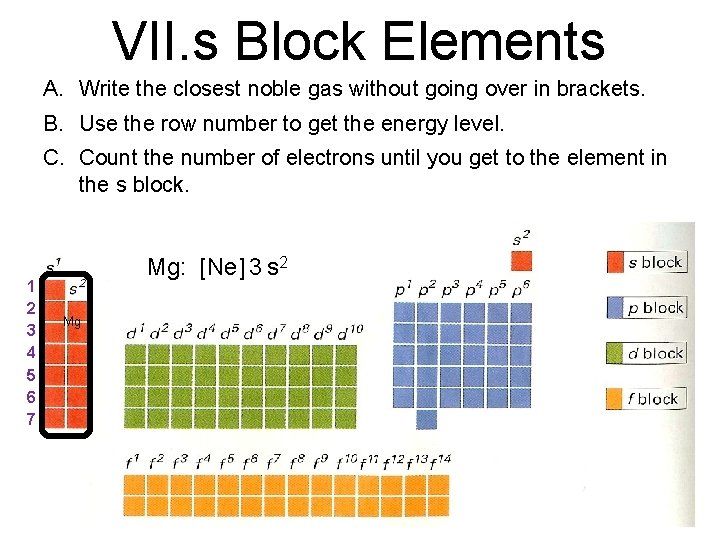
Noble Gas Configuration I What Are Noble Gases

Periodic Table Objectives Ppt Download

Comments
Post a Comment